
Precision Plastic Mould Solutions
2025-06-13 15:23Precision Medical Infusion Needle Manufacturing Specifications
Component Overview
Part Name: Medical Infusion Needle (Class II Medical Device)
Primary Material: PP 9074MED (USP Class VI Certified)
Wall Thickness: 0.7mm ±0.03mm (Critical Flow Path Sections)
Surface Finish: SPI-A2 (Ra ≤ 0.025μm for Fluid Contact Surfaces)
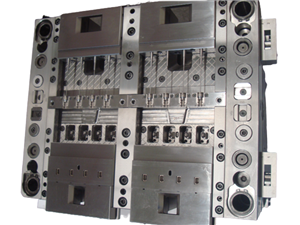
Advanced Tooling Configuration
Mold System: 16-Cavity High-Precision Stack Mold
Core/Cavity Material: Uddeholm M300 Tool Steel
Hardened to 52-54 HRC
Mirror Polished with EDM Texturing
PVD Coated for Corrosion Resistance
Mold Base: NAK80 Pre-Hardened Steel (40-42 HRC)
Integrated Temperature Control Channels
Guided Ejector System with Sleeved Leaders
Hot Runner Technology
System: Moldmaster 8-Point Hot Nozzle System
Individual Zone Temperature Control (±0.5°C)
Balanced Melt Flow Distribution
Quick-Change Nozzle Tips for Different Gate Styles
Gate Type: 0.35mm Hemispherical Hot Tip Gate
Automatic Gate Cutting System
Zero Drool Shut-Off Mechanism
Injection Molding Process
Machine: Toshiba EC-SX 180 All-Electric Press
Clamping Force: 1800 kN
Injection Accuracy: ±0.15% Shot Weight
Repeatability: ±0.002mm Positioning
Process Parameters:
Melt Temp: 220-230°C (Precision Controlled)
Mold Temp: 60-65°C (Water-Oil Hybrid System)
Injection Speed: 120mm/s (3-Stage Profile)
Packing Pressure: 80MPa (Decay Curve Controlled)
Quality Assurance Protocols
Dimensional Verification:
Optical Comparator Inspection (0.001mm Resolution)
CMM Sampling Every 4 Hours (Full GD&T Report)
Functional Testing:
Flow Rate Testing at 23°C ±1°C
Burst Pressure Verification (Minimum 300kPa)
Material Compliance:
FTIR Material Conformity Testing
USP <88> Biological Reactivity Testing
Production Validation
Process Capability: CpK ≥ 1.67 (Critical Dimensions)
Annual Capacity: 18 Million Units (3-Shift Operation)
Cleanroom Standards: ISO Class 7 Molding Environment
Validation Documentation: Full IQ/OQ/PQ Package Available
Regulatory Support
FDA 21 CFR Part 820 Compliance
ISO 13485:2016 Certified Process
REACH & RoHS Material Certification
